- Visibility 12 Views
- Downloads 4 Downloads
- DOI 10.18231/j.ijceo.2020.108
-
CrossMark
- Citation
Is surgical intervention avoidable in congenital nasolacrimal duct obstruction (CNLDO)
- Author Details:
-
Sameera Irfan *
Introduction
Watery eye in the neonatal period or early infancy is a very common problem; infants are brought to the clinic with a watery, red eye and sticky, matted eyelashes, or frank pus in the medial corner of the eye. It is important to decide whether the problem is that of “lacrimation” (an excess production of tears due to ocular irritation), or an “epiphora” (due to a mechanical obstruction in the lacrimal drainage system), or a combination of the two. The common causes of lacrimation that must be looked for are mis-directed eyelashes (trichiasis, distichiasis, entropion lower lid, epiblepharon), conjunctivitis, keratitis and congenital glaucoma.[1]
Epiphora, due to congenital obstruction of the lacrimal drainage system, is a common disorder with a prevalence of 5% to 20% in the pediatric population as mentioned by MacEwen et al.[2] The level of obstruction mostly documented in scientific literature is at the level of Hasner’s valve which guards the outlet of nasolacrimal duct.[3] This results in a troublesome, recurrent unilateral watery eye, but it can occur bilaterally in about 20% cases.[4] It is diagnosed clinically by applying a backward and inwards pressure below the medial canthal tendon with a cotton-tip onto the lacrimal sac, and noting the regurgitation of water, mucus or muco-pus. The lacrimal drainage system can be obstructed at other sites too. In order to understand the exact pathogenesis of this condition, a thorough understanding of the applied anatomy and normal embryological development of the lacrimal drainage system is imperative so that the patients can be managed properly.
The lacrimal drainage system has been described in great detail by many authors;[5], [6], [7] it starts at the punctae (openings), which are round or oval apertures situated on a slight elevation, the lacrimal papilla, at the medial ends of both the upper and lower lids. The upper punctum is slightly medial to the lower one, so that with the eyes closed, they don't overlap each other to obstruct their opening. They are kept patent by a ring of dense fibrous tissue that is continuous with the tarsal plate. Outside this ring of fibrous tissue, fibres of the lacrimal portion of orbicularis muscle (the Horner’s muscle) keep them directed inwards into the lacus lacrimalis, so that the tears can enter the punctae by a capillary action when this muscle contracts during blinking of eyelids.
Each punctum leads into a cannaliculus that runs vertically downwards for 2 mm and then horizontally for 8 mm, sloping towards the medial canthus. The cannaliculi pierce the lacrimal fascia separately and then join together to form the common cannaliculus which is 1-2 mm long. The Horner’s muscle surrounds the cannaliculi thus promoting the onward passage of tears into the lacrimal sac. It opens into the lacrimal sac, about 2.5 mm from the sac’s apex, with an invagination of mucosa forming the Rosenmuller’s valve, that guards this opening, and prevents a backward movement of tears from the sac during blinking. The cannaliculi are lined by non-keratinised stratified squamous epithelium (non-secretory in nature) which is surrounded by a ring of elastic tissue, that makes them distensible, from a normal width of 0.6 mm to 3-fold. Their medial half is covered by the medial canthal tendon (MCT) that anchors the medial ends of eyelids to the medial orbital wall, an important landmark to locate cannaliculi during a surgical dissection.
The lacrimal sac is deeply situated in the lacrimal fossa in the medial orbital wall, formed between the anterior lacrimal crest (frontal process of maxilla) and the posterior lacrimal crest (the lacrimal bone). The sac is enclosed by the lacrimal fascia, which is a part of the orbital periosteum.[7] Loose areolar tissue lies between the lacrimal sac and the fascia, containing a rich plexus of cavernous tissue, so the sac can easily be dissected from the underlying fossa. The sac is closed at the apex, while below, it is continuous with the NLD, a constriction marking the junction between the two. The MCT anchors the medial ends of tarsal plates of both upper and lower eyelids to the anterior lacrimal crest. From the anterior lacrimal crest, the MCT splits into an anterior and a posterior layer. The anterior layer fuses with the dome /apex of the sac, while the posterior layer continues behind the sac, merging with the lacrimal fascia, covers the apex of the lacrimal sac to reach the posterior lacrimal crest.
Also arising from the posterior lacrimal crest is the Horner’s muscle, a fine strip of muscle which is a part of the orbicular oculi, lying behind the lacrimal sac, and passes laterally to split into two slips, that surround the cannaliculi and join the pre-tarsal (Riolan muscle) part of orbicularis oculi muscle. Horner’s muscle helps in creating a vacuum by dilating the dome of the lacrimal sac, so that the tears are transferred easily from the cannaliculi into the sac. With each blink, lacrimal fluid is sucked through the punctae, into the cannaliculi and flows onwards via the common canaliculus into the lacrimal sac. The ability of the canaliculi and lacrimal sac lumen to alter the diameter and create a vacuum is the main lacrimal drainage supporting mechanism, also known as the lacrimal pump; this pump only functions because of the intricate relationship of the Horner’s muscle with all these three structures. The apex of the lacrimal sac is covered by strong fascia and muscle, thus leaving a little room to distend, while below the MCT, the sac is freely distensible.
Busse et al[8] studied the radiological anatomy of tear ducts in neonates at post-mortem, while it was described in more detail by Groell et al[9] by an orbital CT. According to them, the nasolacrimal duct (NLD) is 13-28 mm long (average, 21-22 mm) and 1-4 mm wide. Its upper end, which is continuous with the sac, lies in the orbit and its length depends on the location of lacrimal sac. If the sac is located high, the orbital part of the duct can be up to 10 mm, while if the sac is low, the duct can begin in the bony canal. The usual length of the orbital part is 5-7 mm, and is loosely attached to the underlying periosteum of the lacrimal fossa and can easily be separated from it. The remaining 15-18 mm nasal part of the NLD lies partly in the bony nasolacrimal canal which is only 1 mm in diameter in an infant while 3.3-3.8 mm in an adult. It is created by the lacrimal groove on the maxilla and completed by the lacrimal bone and the inferior concha, and opens into the inferior meatus of the nose. The length of the bony canal was linearly correlated with children's age by Moscato et al,[10] i.e. the bony canal grows with the child’s age. It is small in a newborn so the NLD runs downwards as a membranous tube under the nasal mucous membrane and eventually opens into the inferior meatus of the nose under the inferior nasal turbinate. The mucosal lining of this part of the NLD is closely adherent to bone of the lacrimal groove, forming a muco-periosteium, thus allowing infection to pass easily from the underlying bone as well as the over-lying nasal mucosa to the duct and vice versa. The duct can also easily be compressed by an inflamed nasal mucosa or hypertrophied inferior turbinate. This explains a variety of pathogenic mechanisms causing epiphora in an infant.
The lacrimal sac and the duct are lined by a pseudo-stratified columnar epithelium, that is secretory in nature and covered by cilia. The cilia help in the flow of lacrimal fluid downwards towards the nasal cavity, aided by gravity and by the production of mucins and TFF peptides (trefoil factor family). The columner epithelium contributes to antimicrobial property of the lacrimal fluid by secreting lysozyme, lactoferrin, secretory phospholipase A2, bactericidal-permeability-increasing protein, heparin-binding protein, human -defensins, surfactant proteins, free mucins that engulf microbes invading the tears. At the same time, absorption of lacrimal-fluid components can also occurs.
Underneath the epithelium, the sac and the NLD have a loose connective tissue containing a thin layer of elastic fibres and lymphocytes, sometimes arranged in follicles, that can generate an immune response. A rich venous plexus is situated under the loose connective tissue that continues into the cavernous body of the nasal inferior turbinate. Collagen and elastic fibres between the blood vessels are arranged in a helical pattern and run spirally from the lacrimal sac to the outlet of the nasolacrimal duct, and further contribute bio-mechanically to the onwards flow of lacrimal fluid. Engorgement of these vessels is sufficient to cause NLD obstruction as a part of any nasal pathology (allergy, sinositis).
Regarding the embryological development of the lacrimal drainage system, two diverse hypothesis exist in the scientific literature: According to the first hypothesis, described in great detail by De la Cuadra-Blanco, et al.,[11] and later by Javed, et al.,[12] a single ectodermal fold appears at 32 days of gestation, between the maxillary and fronto-nasal prominences, forming the sulcus of naso-lacrimal groove. A single cord of cells separates from the surface ectoderm at 5th week of gestation and enters this groove. The proximal part of this cord bifurcates to form the cannaliculi, while the remaining forms the lacrimal sac. Its distal end continues to grow downwards into the inferior meatus of the nose to form the NLD.
According to the second hypothesis, described by Cook, et al,[13] the whole system forms from two separate cords of cells that grow in the opposite directions. The main cord of surface ectoderm, buried in the naso-lacrimal groove, forms the lacrimal sac and proximal part of NLD (the orbital part only). It also sends extensions laterally to form both the superior and the inferior canaliculi. A second cord of epithelial cells arises from the nasal cavity and grows upwards towards the first cord of cells to form the remaining nasal part of NLD and, later, fuses with the proximal part.
Whether originating from a single or two cords of cells, the whole tissue eventually canalises in a segmental manner, starting at the 9-10 weeks of gestation. The cavities then coalesce in a haphazard manner to form a continuous tube but few remnants of epithelium persists within the cords forming valve-like folds.
Following developmental anomalies may occur to cause epiphora since birth:
A membranous covering over the puncta, consisting of conjunctival and canalicular epithelium that mostly opens at term, but may persist in a few cases resulting in epiphora at birth. Rarely, punctal agenesis may coexist with occlusion or absence of cannaliculi. In a few cases, punctal and canalicular dysgenesis may occur concurrently with a distal NLD obstruction.
In about 70% newborns, a covering consisting of nasal and nasolacrimal epithelium persists over the nasolacrimal duct outlet, called the Hasner’s membrane, which balloons out into the inferior meatus of nose.[14]
The prevalence of CNLDO along with other developmental anomalies mentioned are much higher in premature infants[15] as compared to full-term babies because full development has not completed in these infants. Children with Down syndrome, clefting syndromes, craniosynostosis, Goldenhar sequence or hemifacial microsomia also have an increased risk for congenital nasolacrimal duct obstruction.
Bone abnormalities, or a stenosis of the inferior meatus leading to a narrowing in the lacrimal drainage system may also occur in few cases.
Since CNLDO is basically due to a delayed canalisation/development of this system, a spontaneous resolution of epiphora is expected to occur by the age of 12 months. A conservative approach during this period should offer a permanent cure, but several studies have reported a highly variable rate (32-95%) of spontaneous resolution of symptoms.[16] This is because of different therapeutic techniques advised by the general ophthalmologists and pediatricians and there is a lack of a standard approach. Recurrent infection of the lacrimal drainage system can result in serious complications like acute / chronic dacryocystitis, acute pre-septal or orbital cellulitis. We conducted this study to find out how efficacious was our technique of sac compression and proper lid cleaning in offering a permanent cure if it was performed properly and for an adequate length of time.
Materials and Methods
A prospective, interventional study was conducted for a period of 6 years, from March 2013 till Dec 2019, at two tertiary care centres, Mughal Eye Trust Hospital, and a private oculoplastics centre in Lahore, Pakistan. A total of 361 consecutive cases were included in the study, but only 338 cases completed the study while 23 dropped out therefore they were excluded from the results. There were 166 females (49.11%) and 172 males (50.88%), Age at the enrolment visit was between 1-47 weeks, with a median age of 23 weeks and a mode of 21 weeks. There were 31 premature babies, (9.2%), born between 30-34 weeks of pregnancy. All cases presented with a watery eye as well as discharge and sticky eyelids. A detailed history regarding the duration of symptoms, previous therapy, sac massage, surgical intervention, as well as the socio-economic status of parents was noted. The general Demographics of 338 cases included in the study are demonstrated in [Table 1].
|
Characteristics |
Number |
% |
|
|
Total Number included |
338 |
||
|
Gender |
Male |
172 |
50.88% |
|
Female |
166 |
49.11% |
|
|
Age at presentation |
Range |
1-47 weeks |
|
|
Median age: |
23 weeks |
||
|
Mode |
21 weeks |
||
|
Premature infants |
Born at 30-34 weeks |
31 |
9.2% |
|
Socioeconomic strata |
Poor / low middle class |
306 |
90.5% |
|
Upper middle class |
32 |
9.5% |
A thorough ophthalmic examination was conducted to find out other causes of a watery eye like a corneal foreign body, trichiasis, distichiasis, entropion, congenital ectropion, epiblepharon, conjunctivitis, keratitis, congenital glaucoma, a nasal pathology as well as the congenital obstruction of punctae, or punctal ectropion. All these cases were excluded from the study. Children older than 12 months age or with a past history of a failed surgical probing, trauma to the nasal region, history of acute dacryocystitis, Down's syndrome, congenital cranio-facial anomalies were also excluded from the study.
A clinical diagnosis of congenital nasolacrimal duct obstruction (CNLDO) was made by pressing on to the lacrimal sac in the medial canthus by a cotton tip and noting the regurgitation of water, pus or muco-pus. At the initial enrolment visit, the primary parameters assessed (Primary Outcome Measures) for the purpose of study were the presence or absence of three clinical signs: epiphora, increased tear meniscus height and presence of mucus / muco-pus on lacrimal sac compression as noted by the primary clinician.
Fluorescein dye Disappearance Test, as described by Mac Ewen and Young,[17] was performed on both eyes to compare the severity of obstruction in bilateral cases or to compare with the normal eye in unilateral cases. A drop of fluorescein dye was instilled into the conjunctival sac without the topical anaesthetic. The lower tear meniscus was observed under the blue filter and its height was noted initially and then after 5 minutes. The test was considered negative (normal) if none or a faint fluorescence of the tear film was remained after 5 min. A thick fluorescein-stained tear meniscus after 5 min was considered a positive test (abnormal). An indeterminate result was considered when a minimally increased tear meniscus was noted with or without any retained fluorescein in the tear film.
An ethical clearance was obtained from the Medical director and the Board of the Trust hospital to conduct the study. All parents were duly counselled and an informed verbal consent was obtained in which the parents agreed to participate in the study and to come for regular follow-ups at 1, 3 and 6 months after the enrolment visit.
Parents, especially the mother of the infant was demonstrated the technique of proper sac compression by a cotton-tip that was directed slightly upwards and inwards below the medial canthal tendon, as shown in [Figure 1].
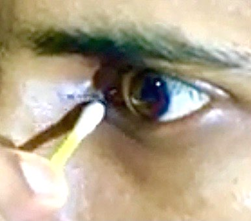
Pressure applied in this direction compresses the whole sac including its apex, and completely empties it. They were also demonstrated the technique to clean the discharge from eyelids and the medial canthus of the eye with a cotton swab. Cases in whom a purulent discharge was noted after sac compression, were prescribed Tobramycin Sulfate 0.3% eyedrops, one drop to be instilled into the eye three times daily for 5 days, after thoroughly emptying the sac and cleaning the discharge. The frequency of sac compression and cleaning routine was according to the severity of patient’s symptoms. Initially, it had to be done every hour for 2 days, followed by 3-5 times, depending upon the level of improvement. The mother was especially informed regarding the recurrent nature of the condition and was instructed not to dis-continue the sac compression and cleaning of eyes at least twice daily, even after the discharge and watering of eye had stopped, till the baby was one year old.
At each follow-up visit, an assessment of the 3 primary parameters: the severity of epiphora, tear-film meniscus height, and discharge on sac compression as well as the FDD Test was performed by the same clinician. The cases that did not show resolution of epiphora or discharge by the age of 10-12 months, were booked for surgical probing of the nasolacrimal duct under a general anaesthesia. Cases that did not attend the final follow-up visit at 6 months after enrolment (the drop-outs) were excluded from the study.
Statistical analysis: The Demographic variables were described by a mean and median for the continuous data; the percentages were calculated for the categorical data. One Way ANOVA test was performed and a P-value less than 0.05 was considered statistically significant.
Results
Out of the 361 cases initially enrolled in the study, 23 cases did not attend the final 6 months follow up visit, therefore they were considered as drop-outs and were excluded from the final analysis of study results. The general characteristics of the 338 cases who attended the final follow-up and completed the study, and are shown in [Table 2].
|
Characteristics |
Number of cases |
% |
|
|
Laterality |
Unilateral |
305 |
90.3% |
|
Bilateral |
33 |
9.7% |
|
|
Mode of Presentation |
Primary |
41 |
12.13% |
|
Secondary (referral) |
297 |
87.86% |
|
|
Used Antibiotics |
Primary cases |
none |
|
|
Secondary cases |
297 |
87.86% |
|
|
Previous sac massage |
Primary cases |
none |
|
|
Secondary cases |
Crigler massage = 221 |
62.4% |
|
|
Medial Sac compression= 86 |
25.4% |
||
|
Symptoms |
Epiphora |
338 primary & secondary cases |
100% |
|
Discharge |
Primary 41 cases = none |
12.13% |
|
|
Secondary cases: Mucoid = 80 |
23.66% |
||
|
Mucopurulent = 217 |
64.2% |
||
|
FDDT |
Positive |
338 |
100% |
|
Tear meniscus height |
increased |
338 |
100% |
Laterality
Only 33 cases (9.7%) had a bilateral involvement, while the rest of 305 cases (90.3%) had a unilateral involvement.
Mode of presentation
Primary cases that had not used any prior therapy were only 41 in number (12.13%); they presented early, within the first two weeks of the onset of epiphora, with no discharge in the eye and regurgitation of mucoid-water on sac compression. The remaining 297 cases (87.86%) presented secondarily and were already using antibiotic eyedrops prescribed either by the paediatrician or a general ophthalmologist elsewhere for recurrent discharge for 1-5 months. They were also performing either the Crigler massage, 211 cases (62.4%), while 86 cases (25.4%) were performing medial sac compression once or twice daily and for a short while only. Recurrent symptoms were noted in all these secondary cases.
Socioeconomic strata
Out of 338 cases, 306 cases (90.5%) belonged to the poor or lower socio-economic strata of the society, while only 32 cases (9.5%) belonged to the upper middle class, with a highly significant p value of <0.0001.
Clinical signs at presentation
Epiphora was the main presenting complaint in all 338 cases (100%); it was the only complaint in all primary 41 cases (12.13%) that presented within 1-2 weeks after birth. The 297 secondary cases presented with recurrent discharge in the eye in addition to epiphora. It was noted to be muco-purulent in 217 cases (64.2%), present either in the medial canthus of the eye or on lacrimal sac compression; mucoid discharge only on sac compression was noted in 80 cases (23.66). Fluorescein dye Disappearance Test was positive (abnormal) in all cases, with a thick tear meniscus.
The results of serial followups at 1, 3 and 6 months are demonstrated in [Table 3]. Treatment was classified as successful when all three primary parameters (epiphora, increased tear meniscus and mucous discharge on sac compression) were absent and FDDT had become negative. The results were analysed separately for primary cases (which had no prior therapy and presented early, soon after the onset of epiphora) and secondary cases (which presented at an average age of 5 months and had already used topical antibiotics and sac massage).
|
Parameter |
Presentation Mode |
1 month Total cases 338 |
3 months Total cases 296 |
6 months Total cases % 338 |
Overall Final Result 338 cases |
|
Epiphora |
Primary: 41 cases |
Reduced :32, Absent : 9 |
Recurrence:20, Absent: 17 |
Recurrence: 3 (7.31%), Absent; 38 (92.68%) |
Absent in 316 cases (38+278) = 93.49% |
|
Secondary 297 cases |
Reduced: 297, Absent: none |
Reduced: 135, Absent: 124 |
Recurrence:19 (6.40%), absent: 278 (93.60%) |
Present in 22 cases (3+19) = Failure of 6.50% |
|
|
Tear meniscus height |
Primary |
High: 41 |
High: 20, Normal: 17 |
High: 3 (7.31%), Normal: 38 (92.68%) |
|
|
Secondary |
High 297 |
High: 135, Normal 124 |
High: 19(6.40%), Normal:278 (93.60%) |
||
|
Discharge |
Primary |
Absent: 41 |
Present: 20, Absent: 17 |
Present: 3 (7.31%), Absent: 38 (92.68%) |
|
|
Secondary |
Present: 297 |
Present: 135, Absent: 124 |
Present: 19 (6.40%), Absent: 278 (93.60%) |
||
|
FDDT |
Primary |
Positive 41 |
Positive 37 |
Positive: 11 (26.82%), Negative:30 (73.17%) |
Overall negative in 300 cases (88.75%) |
|
Secondary |
Positive 297 |
Positive 259 |
Positive:26 (8.75%) Negative: 271(91.24%) |
Overall Positive in 38 cases (11.24%) |
At the first follow-up visit, which was one month ofter starting the therapy, all 338 cases attended. Out of the primary 41 cases, 32 had reduced epiphora, while it had cleared up in 9 cases. Out of the secondary 297 cases, epiphora had reduced but not yet cleared up. There was a marked reduction in purulent discharge in all secondary cases, and was noted intermittently. FDDT was still positive in all primary and secondary cases. The parents were strictly instructed to continue the sac compression and cleaning of eyelids, even if watering or the discharge had totally disappeared, till the baby was one year old, in order to avoid recurrence and surgical intervention.
The 3-month follow-up was attended by 296 cases (37 primary + 259 secondary= 87.57%); epiphora was present intermittently as well as discharge on sac compression in 20 primary cases as they had left sac compression when the symptoms had improved, despite strict instructions to continue. FDDT was positive in all these cases. Similarly, in 135 secondary cases, epiphora and discharge re-appeared after an interval of 2-3 weeks once the mother had stopped sac compression.
At the final 6 months follow up, all 338 cases attended. There was a complete resolution of symptoms and absence of discharge on sac compression in 38 primary cases (92.68%) and 278 secondary cases (93.60%). There was not a significant difference between the two groups (p > 0.05). A complete resolution of symptoms and absence of discharge on sac compression was present in 316 cases (93.49%); this was considered a successful outcome. The FDDT was still slightly positive after at 5-10 minute interval in 16 cases though they had a complete resolution of symptoms and no regurgitation of any fluid on sac compression and were included in the 316 cases with a successful outcome. This indicated that the canalisation of the system had not yet completed in these cases and they were instructed to continue with the sac compression for another one-two months.
Recurrent epiphora and purulent discharge was present in 22 cases, (3 primary and 19 secondary= 6.50%), along with a positive FDDT. They were considered as a failure of conservative management. The total number of cases in which the FDDT was positive at the end of study were 38 (16 + 22, 11.24%). The failed cases were due to a poor compliance to a continued conservative therapy and they had to be booked for surgical probing. Out of these 22 cases, 5 had a bilateral epiphora, (10 cases/eyes) while the remaining 12 had a unilateral problem. At probing, in 19 cases, a blockage was found at the site of junction of the lacrimal sac with the NLD, while in 3 cases, a common cannalicular block was found as well.
A complete resolution of symptoms and signs was significantly higher in unilateral cases than bilateral (p=0.001). There was no significant differences amongst genders (p=0.684), or amongst primary versus secondary cases (p=0.062) as they all responded to correct conservative management.
Discussion
Epiphora due to CNLDO is a common problem encountered in 1 in 9 newborns, that tends to undergo spontaneous resolution by the age of 12 months as mentioned in studies by Sathiamoorthi et al., and Schnall BM.[18], [19] There is a combination of factors which contribute to the patency of NLD and a spontaneous resolution of epiphora. Firstly, the central cavitation of epithelial core, which starts from the eighth week of intrauterine life and should complete at birth. Secondly, an elongation and volume expansion of the NLD mostly occurs in the first six months after birth and continues till the age of 1 year; therefore, all of these cases respond well to conservative therapy initially, but a failure to continue with the therapy till the infant has become 1 year old, results in recurrent purulent discharge and a need for surgical intervention (as in our 22 cases, 6.5%). Thirdly, an increase in the hydrostatic pressure of the fluid column within the NLD contributes to rupturing of a persistent Hasner’s membrane. Therefore, a CNLDO is more common in premature babies, as noted by Sathiamoorthi et al; this is because the full development of the lacrimal drainage system has not occurred yet, and is mostly bilateral (as in our study all 33 premature babies had a bilateral problem). In full-term babies, a CNLDO is mostly due to a delayed canalisation of the nasolacrimal system.
In this study, all 41 primary cases (12.13%) presented early i.e. within 1-4 weeks of birth and the onset of epiphora, and on lacrimal sac compression, regurgitation of a small amount of a clear lacrimal fluid was noted. FDDT was positive in all these cases. In contrast, the 297 secondary cases (87.86%) presented late, between the age 21-23 weeks after the onset of epiphora and with a recurrent purulent discharge. The infection was mostly of a low grade. All of them had been using antibiotic eyedrops for weeks as well as performing sac massage, though infrequently (Crigler's as well as medial sac compression). These observations highlight certain important points regarding the pathogenesis of CNLDO:
Firstly, an incomplete canalisation of NLD at birth or a persistence of Hasner’s membrane results in stasis of lacrimal fluid in the lacrimal sac; stasis is the main factor that promotes bacterial growth in the warm, moist environment of the lacrimal sac and formation of a purulent discharge. The inflammation spreads along the lacrimal sac and the whole extent of NLD as both these structures are surrounded by a rich venous plexus. The reason why our primary 41 cases only had an epiphora and no purulent discharge was that they presented early before bacterial proliferation had occurred, and responded well to sac compression technique alone without the need for topical antibiotics. A few of them may had some degree of canalicular stenosis too, (that could only be proved by probing, which we didn't perform at such an early stage), which also improved over time by the hydrostatic pressure exerted by the regurgitated fluid from the lacrimal sac compression and a resultant dilation of the common canaliculus. On the other hand, our 297 secondary cases (87.86%) presented late. Stasis of lacrimal fluid in the lacrimal sac promoted bacterial growth with formation of purulent discharge. In a study by Xiao et al,[20] the bacterial species most frequently cultured from the lacrimal fluid samples obtained at probing were Streptococcus (50%), Haemophilus (21.1%) and Neisseriae (13.2%). We used Tobramycin eyedrops which has a broad spectrum and was considered safe for our cases which needed it as they were mostly 3-5 months old.
Secondly, the injudicious use of antibiotic eye drops has no bearing on the resolution of epiphora, as the primary cause is an obstruction of outflow. There is no evidence in scientific literature about the efficacy of antibiotics in the resolution of CLNDO.[21] Therefore, all our primary cases responded to therapy without the need for antibiotics. The secondary cases had been using topical antibiotics for weeks and still developed recurrent discharge because a prolonged antibiotic use facilitates the growth of resistant bacteria in an already inflamed lacrimal sac with static lacrimal fluid. In our cases, tobramycin eyedrops was administered for only 5 days in cases which demonstrated a strong clinical evidence of infection (purulent discharge, inflamed eyelids) and orally too in cases of acute dacryocystitis, so as to prevent complications like perseptal / orbital cellulitis as a result of spread of infection through the thin lamina papiracea to adjacent orbital tissues.
Thirdly, most studies implicate a persistent Hasner’s membrane as the commonest cause of CNLDO. But once the membrane has ruptured from a raised hydrostatic pressure by sac compression, recurrent symptoms should not occur. We believe that either a delayed canalisation of the NLD could be a more frequent cause of epiphora at birth rather than a persistent Hasner’s membrane or the formation of fibrous adhesions within the lacrimal sac and the duct following inflammation might be responsible for recurrent symptoms. This might be the case in all our premature babies and recurrent secondary cases, where repeated infection by resistant bacteria (due to misuse/over-use of topical antibiotics) led to the formation of inflammatory adhesions, and obstruction of NLD.
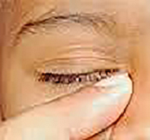
Fourthly, most of our secondary cases (87.86%) had recurrent purulent discharge and a troublesome epiphora despite having performed sac massage by the Crigler's technique, as advised by their paediatrician or the general ophthalmologists whom they visited initially. This technique was introduced by Crigler LW in 1923,[22] and involves placing a finger over the medial canthus (overlying lacrimal sac and common canaliculus) to block the exit of fluid towards the eye, while firmly stroking downwards alongside the lateral nasal wall, as demonstrated in [Figure 2]. This was done 5-10 times/day, with the aim to increase hydrostatic pressure within the NLD. This has been the most popular technique amongst the general ophthalmologists and had variable results. Many clinical studies have questioned the clinical efficacy of this manoeuvre,[23], [24] Stolovitch et al.[25] demonstrated a success rate of 56% in children less than 2 months age, 46% in children aged 2 to 6 months, and only 28% in children older than 6 months. The main reason for the reduced success by this technique is that the pressure exerted by the finger over the medial canthus does compress the sac partially to increase the hydrostatic pressure of fluid column in NLD that helps in rupturing the Hasner’s membrane, but it does not completely empty the lacrimal sac lying deep within the tiny lacrimal fossa. Therefore, stasis of residual lacrimal fluid promotes repeated infections. Similarly, the repeated downward strokes applied along the bony lateral nasal wall do not serve any purpose as the pressure applied cannot be transmitted to the NLD lying deep within the bony lacrimal canal.

Fifthly: we demonstrated the correct technique of sac compression (not sac massage) in all our cases by using a cotton-tip! The dimensions of the lacrimal fossa in an infant are 4-8 mm in width, 15 mm in height, and 2 mm in depth, and are much less in a premature baby. A cotton-tip is small enough to fit into a 4-8 mm wide lacrimal fossa rather than the finger-tip of an adult, which is much larger than the dimensions of a small lacrimal fossa of an infant, as shown in [Figure 3]. The pressure with the cotton-tip was directed slightly inwards and downwards below the medial canthal tendon to compress the sac and empty it fully, as demonstrated in [Figure 4]. Then the discharge was cleaned from the eyelids and the medial canthus of the eye with a cotton swab. This was done frequently initially, perhaps every 2 hourly, and especially prior to instillation of antibiotic eyedrops (in secondary cases with a purulent discharge). The discharge was more, initially, as the inflammation spreads easily along the whole lining epithelium of the lacrimal sac and the NLD, with exudation of fluid from the engorged vascular network around the sac, in addition to the incoming lacrimal fluid. As the inflammation gets controlled by antibiotics, the severity of exudation and discharge reduces and it became clear, watery, so the frequency of sac compression was reduced to 2-4 times per day.

The sixth important point/lesson learnt from this study was that the FDDT is an important predictor of an incomplete / delayed canalisation of the lacrimal drainage system. This test has been shown to have a 90% sensitivity and 100% specificity for CNLDO by MacEwen and Young while in another study by Bowyer et al. it had a 76% sensitivity and specificity.[17], [26] All our cases responded well to this approach at the first follow-up visit and demonstrated an improvement in all the three clinical parameters. However, FDDT was still slightly positive, indicating the blockade had not yet cleared up fully. Therefore, they were instructed to continue with the lacrimal sac compression even after the resolution of symptoms, till the baby was 1 year old; this had been stressed upon in other studies too.[27] However, the cases that discontinued the therapy sooner, developed recurrence of epiphora as well as purulent discharge at the 2nd and the final follow-up visit. FDDT was positive in all these cases. Therefore, in our study too, it accurately predicted the cases in which the blockade had not fully resolved and helped parents understand the need to continue with the sac compression and cleaning therapy.
The seventh important point noted in our study was that in the 22 cases (6.50%) which needed a surgical probing, a blockage of NLD was noted, in 19 cases, at the site where the orbital part of NLD joined the nasal part lying in the bony nasolacrimal canal. Anatomically, this is the narrowest point of NLD, while embryologically, this is the last point of fusion of the two ectodermal buds and is the last to canalise as already mentioned. Recurrent inflammation had resulted in the formation of permanent fibrotic stricture at that anatomically narrow site resulting in a permanent occlusion of NLD. This finding must be looked into in future studies as it has not been mentioned in the scientific literature as an important cause of recurrent symptoms in CNLDO. In 3 cases, a stenosed / blocked common canaliculus was detected at probing; this may have been due to fibrous adhesions from recurrent inflammation spreading from the lacrimal sac. Congenital common canalicular obstruction is extremely rare (7.3%) and is associated with punctal stenosis or atresia in patients with anophthalmos or microphthalmos.[28]
The eighth important point noted was that a complete resolution of symptoms and an absence of discharge on sac compression at the end of study was present in 316 cases with a successful outcome of 93.60% (p: < 0.001). There was not much difference in the outcome between primary and secondary cases (p= 0.062). Compliance of parents to continue with the proper technique (that was demonstrated at each visit), till the infant was a year old even after the symptoms had cleared up, were the two main factors that ensured such a high success rate in our cases. This final successful outcome was much higher than quoted in other studies and was merely because of guiding the parents properly and repeatedly at each visit. This added strength to our study, in addition to the facts that it was prospective, with a large sample size and included a diverse population (as most cases were referred) from all over the country. In the 338 cases who completed our study, not much gender difference was found, with 166 females (49.11%) and 172 males (50.88%); this was similar to other studies. Our most cases (306=90.5%) belonged to the lower socioeconomic strata, with a highly significant p: < 0.0001, indicating that a bad personal hygiene and poor general health of newborns may be the implicating factor.
The relative shortcoming of this study was that it was not a randomised controlled trial. As 211 cases (62.4%) in our study had already been performing Crigler massage while 86 cases (25.4%) had performed a medial sac compression, infrequently and for a short time, and were still having recurrent epiphora and purulent discharge; therefore, an RCT was deemed unnecessary. We guided our patients’ caretakers to perform only the medial sac compression and lid cleaning meticulously if they wanted to avoid surgery, and the parents complied.
Conclusion
It must be remembered that the principle of an affective conservative approach is to prevent recurrent infection by keeping the lacrimal sac empty at all times, till the lacrimal drainage system is fully canalised. Hence, every clinician must demonstrate the correct technique of sac compression and cleaning of eyelids and must ensure that the instructions are properly understood by the parent/care-taker; they need to be reminded at each visit to continue with the sac compression and cleaning at least once or twice daily till the baby is one year old, if they want avoid a subsequent surgical intervention. A proper and repeated guidance to parents/caretakers is the key to a successful outcome.
Injudicious use of topical antibiotics should be avoided.
In view of a high successful outcome of this study, we recommend that the term “sac massage” is a misnomer, and it should be replaced by “Sac Compression ! ”
Source of Funding
No funding was required as it was a clinical research project.
Conflict of Interest
The author declare no conflict of interest.
References
- A J Swampillai, T F McMullan. Epiphora. Br J Hosp Med 2012. [Google Scholar] [Crossref]
- C J Macewen, J D H Young. Epiphora during the first year of life. Eye 1991. [Google Scholar] [Crossref]
- G T Lueder. Treatment of nasolacrimal duct obstruction in children with trisomy 21. J Am Assoc Pediatr Ophthalmol Strabismus 2000. [Google Scholar] [Crossref]
- S E Olitsky. Update on Congenital Nasolacrimal Duct Obstruction. Int Ophthalmol Clin 2014. [Google Scholar] [Crossref]
- C Zhang, Q Wu, Y Cui, G Yu. Anatomy of nasolacrimal canal in congenital nasolacrimal duct obstruction. Acta Ophthalmol 2015. [Google Scholar] [Crossref]
- A H Weiss, F & Baran, J Kelly. Congenital nasolacrimal duct obstruction. Arch Ophthalmol 2012. [Google Scholar]
- E Tatlisumak, A Aslan, A Comert. Surgical anatomy of the nasolacrimal duct on the lateral nasal wall as revealed by serial dissections. Anat Sci Int 2010. [Google Scholar]
- H Busse, K M Müller, F Osmers. The radiological anatomy of the tear ducts in neonates. Rofo 1977. [Google Scholar]
- R Groell, G J Schaffler, M Uggowitzer, D H Szolar, K Muellner. CT-anatomy of the nasolacrimal sac and duct. Surg Radiol Anat 1997. [Google Scholar] [Crossref]
- E E Moscato, J P Kelly, A Weiss. A Developmental anatomy of the nasolacrimal duct: Implications for congenital obstruction. Ophthalmol 2010. [Google Scholar]
- C de la Cuadra-Blanco, M D Peces-Pena, L Janez-Escalada, J R Merida-Velasco. Morphogenesis of the human excretory lacrimal system. J Anat 2006. [Google Scholar] [Crossref]
- M J Ali, H Kakizaki. Embryology of the Lacrimal Drainage System. Principles and Practice of Lacrimal Surgery 2018. [Google Scholar] [Crossref]
- C S Cook, V Ozanics, F A Jakobiec, W Tasman, E A Jaeger. Prenatal development of the eye and its adnexa. Duane’s foundations of clinical ophthalmology 1994. [Google Scholar]
- D Sevel. Development and congenital abnormalities of the nasolacrimal apparatus. J Pediatr Ophthalmol Strabismus 1981. [Google Scholar]
- S H T Lorena, Jo A F Silva, M J Scarpi. Congenital Nasolacrimal Duct Obstruction in Premature Children. J Pediatr Ophthalmol Strabismus 2013. [Google Scholar] [Crossref]
- C Petris, D Liu. Probing for congenital nasolacrimal duct obstruction. Cochrane Database Syst Rev 1991. [Google Scholar]
- M B Kashkouli, H Mirzajani, M Jamshidian-Tehrani, S Shahrzad, M S Sanjari. Fluorescein Dye Disappearance Test: A Reliable Test in Assessment of Success After Dacryocystorhinostomy Procedure. Ophthal Plast Reconstr Surg 2015. [Google Scholar]
- S Sathiamoorthi, R D Frank, B G Mohney. Incidence and clinical characteristics of congenital nasolacrimal duct obstruction. Br J Ophthalmol 2019. [Google Scholar] [Crossref]
- B M Schnall. Pediatric nasolacrimal duct obstruction. Curr Opin Ophthalmol 2013. [Google Scholar]
- X Y Zheng, B N K Choy, M M Zhou, C P Shi, Z Y Zhao. Lacrimal sac bacteriology and susceptibility pattern in infants with congenital nasolacrimal duct obstruction in the 1st year of life: a cross-sectional study. BMC Pediatr 2020. [Google Scholar] [Crossref]
- H Kakizaki, Y Takahashi, S Kinoshita, K Shiraki, M Iwaki. The rate of symptomatic improvement of congenital nasolacrimal duct obstruction in Japanese infants treated with conservative management during the 1st year of age. Clin. Ophthalmol 2008. [Google Scholar]
- L W Crigler. The treatment of congenital dacryocystitis. JAMA 1923. [Google Scholar]
- O Karti, E Karahan, D Acan, T Kusbeci. The natural process of congenital nasolacrimal duct obstruction and effect of lacrimal sac massage. Int Ophthalmol 2016. [Google Scholar] [Crossref]
- . Pediatric Eye Disease Investigator Group. Resolution of CNlDO with Nonsurgical Management. Arch Ophthalmol 2012. [Google Scholar]
- C Stolovitch, A Michaeli. Hydrostatic Pressure as an Office Procedure for Congenital Nasolacrimal Duct Obstruction. J Am Assoc Pediatr Ophthalmol Strabismus 2006. [Google Scholar] [Crossref]
- J D Bowyer, C Holroyd, A Chandna. The use of the fluorescein disappearance test in the management of childhood epiphora. Orbit 2001. [Google Scholar] [Crossref]
- Y Takahashi, H Kakizaki, W O Chan, D Selva. Management of congenital nasolacrimal duct obstruction. Acta Ophthalmol 2009. [Google Scholar] [Crossref]
- M P Schittkowski, R F Guthoff. Results of lacrimal assessment in patients with congenital clinical anophthalmos or blind microphthalmos. Br J Ophthalmol 2007. [Google Scholar] [Crossref]
